The long road to curing TB, together
Ufrieda Ho

Photo by Damian Schumann
A centuries-old disease still stalks humankind today. What is becoming increasingly clear, though, is that the answer may lie in researchers, scientists and patients first finding their common humanity.
Humans have travelled a long journey with tuberculosis (TB) – since the first centuries after people abandoned nomadic lifestyles to settle down. But after 9 000-odd years of known contact with the bacterium, exactly how to leave TB in our past still eludes scientists.
Finding solutions to curbing TB in our modern era demands equal parts scientific and technological advancement, advocacy to raise the profile of the disease burden, and acute awareness of the societal inequalities that make the poorest communities the most vulnerable.
The TB disease burden
Prof Gerhard Walzl, clinician scientist and head of Stellenbosch University’s (SU’s) Immunology Research Group, believes that TB is still far from being eradicated, having claimed the lives of 1,5 million people in 2020, according to the World Health Organization.
“We have huge problems with TB, starting with its diagnosis. The diagnostic process is too complicated for the settings in which TB occurs in South Africa, which are resource-poor areas in which our healthcare system cannot deal effectively with the infection load,” Walzl says. For context, he adds that 85% of the South African population is reliant on the country’s ailing public healthcare system.
Other challenges he mentions include long clinic queues, staff shortages, the poor sensitivity of sputum tests, bottlenecks in sending samples off to laboratories for testing, and the need for a follow-up clinic visit for test results to be acted upon.
“We know that about 25% of people just don’t come back for their test results. And many more don’t ever get diagnosed and so continue infecting others. That’s why we talk about TB’s ‘missing millions’,” he says.
Prevention mechanisms have also become dated, Walzl points out. The bacillus Calmette-Guérin (BCG) vaccine, while still in use, is over 100 years old and has been shown to mainly protect against disseminated forms of TB, predominantly in children under the age of five years. Also, it does not ensure lifelong immunity.
Yet another barrier to TB eradication, he says, is that TB treatment regimens still require daily antibiotic treatment for a full six months. Many people fail to complete their treatment course once it starts to make them feel better. To make matters worse, incomplete treatment is adding to the problem of a rise in drug-resistant TB strains.
“For the last 10 years or so, we have seen a worrying increase in drug-resistant TB, extensively drug-resistant TB, and totally drug-resistant TB, which is an untreatable form of tuberculosis,” he says.
Turning the tide on TB will come down to the use of multi-pronged approaches, Walzl believes. He says the “pleasant noises” made about taking TB seriously need to translate into balanced funding, investment in research, and the political will to put TB on everyone’s agenda. According to him, truly tackling TB will demand vigour similar to that which drove the COVID-19 pandemic response in recent years and the HIV response in the late 1990s and early 2000s, which both dominated public attention.
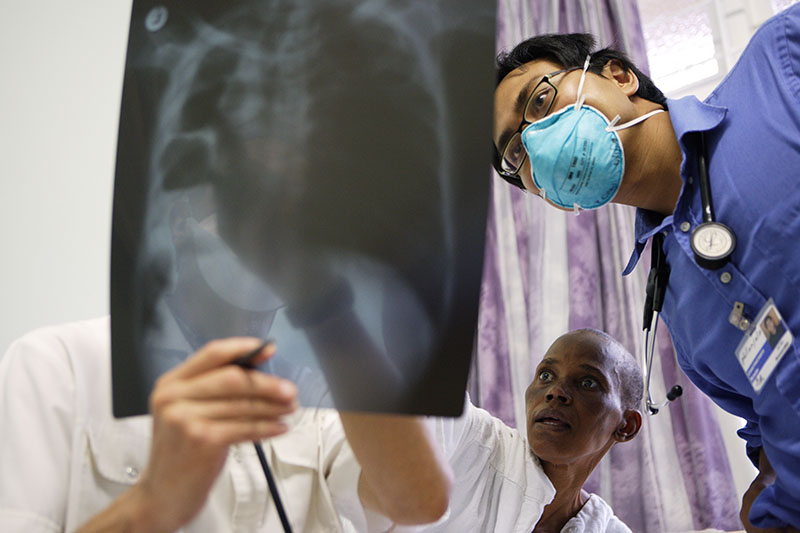

Diagnosing TB requires looking at a patient’s clinical picture, as well as the results of certain tests. Here, an unidentified patient and doctors discuss the results of a chest X-ray to determine treatment. Photos by Damian Schumann

Prof Gerhard Walzl | Photo by Stefan Els

Photo by Damian Schumann
Hope in the dark
Walzl also highlights some promising leaps forward: “There have been major successes in molecular diagnostics following the use of the polymerase chain reaction (or ‘PCR’) test that can detect TB genetic material in a sputum sample and deliver a result within two hours. This means you don’t need to culture the bacteria. The PCR-machines have also become smaller and smaller so they can be placed in clinics. We just need to make them more widely available.
“We have also seen new drugs that promise to shorten treatment time and we have a number of vaccines being trialled right now. Even though the trials are expensive and take a long time to be completed, it’s still significant,” he adds.
Feeding his optimism further is the strong foundational knowledge in South Africa and the continent at large, along with growing collaboration.
“Within the University, we have a wide portfolio of TB expertise, ranging from that in paediatric tuberculosis, adult tuberculosis, microbiology, vaccine testing, drug development and immunology to that in genetics. We also do a lot of social impact work that tries to inform the population about symptoms and risks, and foster a greater understanding of the problem,” Walzl says.
Cracking the code
For Prof Marlo Möller, head of the TB Host Genetics Research Group in SU’s Division of Molecular Biology and Human Genetics, the latest genetic research is revealing previously unimagined clues that deepen the understanding of the TB bacterium and the complex pathways of TB infection in its hosts. In turn, this gives clues to susceptibility, immunity, and how and why different patients metabolise and respond to treatment differently.
“Scientists have been trying to cure TB for a long time, but we want to speed things up. With genetic research, even in the case of a complex disease like TB, we are finding out things we didn’t know before.
“For instance, there is one genetic region that has been coming up during testing in several populations, and it’s actually one of the first genetic associations linked to TB. This is the human leukocyte antigen (or ‘HLA’) region. In these immune genes, we get thousands of single-base-pair changes in the DNA, so it’s really very complex,” she says.
The HLA region, found on chromosome 6, regulates immunity and plays a role in assessing the success of organ transplants. HLA is also an important determinant of the outcome of TB recovery and survival.
“It’s a very complex region of the genome because the HLA genes are many, they’re small and they differ a lot between individuals and in different populations. So that’s one aspect that we would definitely like to investigate further,” says Möller.
Möller and her team's work have already enlarged biobanks in South Africa so that meta-analysis can now be undertaken between South Africans and other Africans. Collecting the genetic samples of more people for libraries of genetic data is a core building block for advancing personalised medicine of the future. This is because metadata as reference allows the increased refining of genetic testing, which in turn heightens the possibility of targeted treatment and support for individual patients.
“Improving our understanding of the human genome and how it works means that at some point, we will be able, for instance, to predict who will get TB and get sick.
“TB genetics is, of course, complex, because we have evolved together for so long. It’s not just one gene contributing to an outcome; we’ve seen that several immune genes can contribute,” Möller says.
While cracking the codes of this complexity is certainly at the forefront of cutting-edge scientific research, Möller is cognisant of the fact that any research in this regard should ultimately be to the tangible benefit of the communities that are most affected by TB. Her division has set up programmes to ensure that their key research results are taken back to the communities in which they work. “I love those meetings [between researchers and community members] because you have such great conversations,” she says.

Prof Marlo Möller | Photo by Stefan Els

Prof Novel Chegou | Photo by Stefan Els
Better testing tools not the sole solution
South Africa’s TB burden is weighed in the 60 000 known deaths that resulted from this disease in 2021. It’s a chilling wake-up call, says Prof Novel Chegou of SU’s Department of Biomedical Sciences. “TB is still the disease of the poor,” he says in explanation of why it is still not being sufficiently prioritised. This is what drives Chegou’s focus on tools, treatments and services that improve access to TB tests, as well as their ease of use. “We are working on finding biomarkers [biological indicators] in bodily fluids other than sputum, like blood or urine, because sometimes people can’t give a sputum sample or the TB is not present in the lungs. So, we are trying to develop more sensitive TB tests and bring down the cost of the test kits,” he says. Encouragingly, Chegou says they already have nine patent applications. Protecting the intellectual property is not for the purposes of commercial gain, he says, but to ensure that the products developed truly serve the interests of the most vulnerable. “In the next 10 years, I think we’re going to have a lot of tools for testing and understanding TB. What we’ll need more than tools, however, is commitment from politicians and policymakers. Even if we have the tools, if those who are supposed to make the laws [related to their use, distribution and access] don’t make them, then the tools are of no help to anyone,” he says. It’s a remark that reconnects the fight to eradicate TB with its original intent: to support people, not profits or politics.