Breakthrough work on microclots may explain long COVID
Wiida Fourie-Basson
Original photo | Dr Chantelle Venter
Proteomics. Genomics. Systems biology. Machine learning.
Researchers at Stellenbosch University (SU) are using all possible tools to figure out how exactly the coronavirus disease (COVID), caused by the SARS-CoV2 virus, develops into long COVID, which affects an estimated 1,2 million South Africans.
Leading this collaborative, multidisciplinary effort is Prof Resia Pretorius, distinguished professor and head of the Department of Physiological Sciences at SU.
The likely suspects
In 2021, Pretorius made a breakthrough discovery when she discovered insoluble microclots in blood samples from individuals suffering from long COVID. For the first time, it made sense why some individuals, long after having contracted the virus, were still complaining of constant fatigue, brain fog, muscle pain and heart palpitations. Pretorius and a team of specialists and clinical collaborators have, to date, provided consistent evidence that COVID-19 is not a respiratory disease, as originally thought, but a complex multisystemic disease that causes widespread organ and tissue damage.
The latest report on long COVID from the United States Government Accountability Office (GAO), published in March 2022, identifies four potential causes of long COVID that need further investigation: the body’s autoimmune response, organ damage, microclots and the persistence of the virus in the body. Except for the latter, says Pretorius, they have thus far provided evidence confirming all these causes.
The journal Science recently listed microclots as one of the leading theories explaining the pathology of long COVID.

Dr Resia Pretorius | Photo by Stefan Els

Original photo | Dr Chantelle Venter
Listen to Dr Resia Pretorius providing a snapshot of her work
Long COVID presents as a collection of lingering symptoms that last 90 days or more after patients have recovered from acute COVID-19 infection. Some of the symptoms include fatigue, difficulty breathing, shortness of breath during daily tasks, joint pain and chest pain. By March this year, long COVID was estimated to have affected at least 45% of the 2,7 million South Africans who had survived the SARS-CoV-2 infection. This means that over 1,2 million South Africans likely required further medical support for this very poorly understood condition. At the same point in time, in the United States of America, an estimated 7 million to 23 million people were likely affected. (Source: United States Government Accountability Office, March 2022)
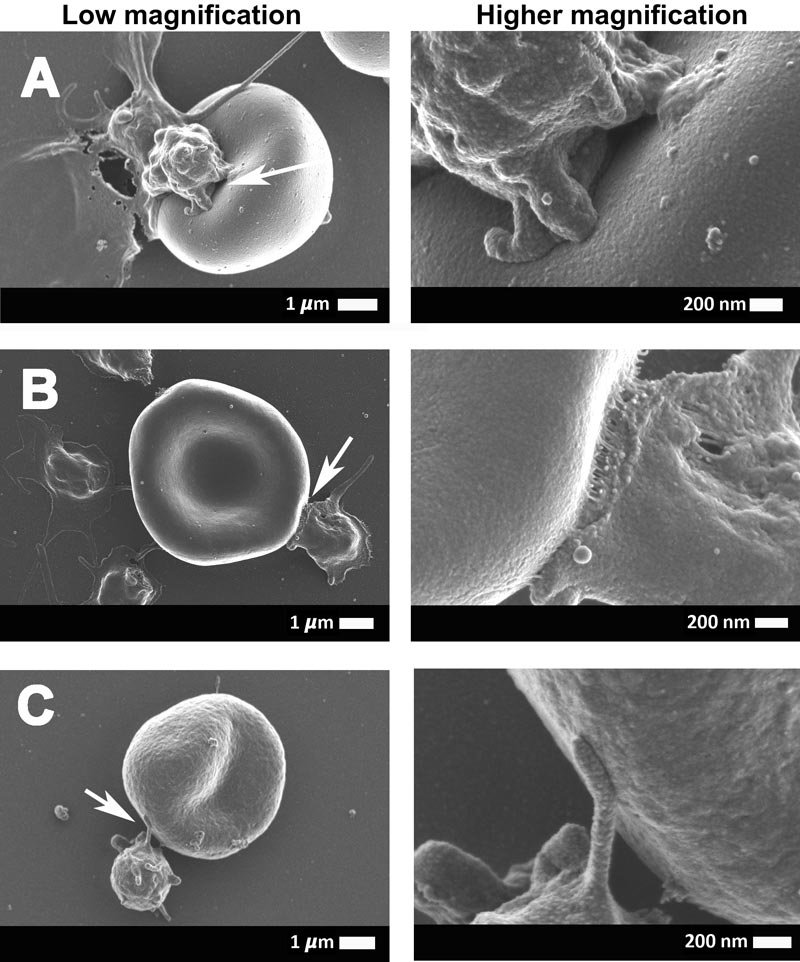
These electron micrographs show the interaction between the erythrocytes (red blood cells) and platelets of COVID-19-positive patients. The arrows point to the area that was focused on.

Dr Resia Pretorius in her laboratory looking at microclots - now researched as one of the most likely causes of long COVID.
Making the link between blood clotting and long COVID
In 2020, when the dominant thinking was still that COVID-19 is a respiratory disease, Pretorius worked with two specialists in private practice at Mediclinic: Dr Jaco Laubscher, a vascular internist, and anaesthesiologist Dr Johan Lourens. The trio had a novel hypothesis: COVID-19 is a vascular disease, not a respiratory one. If they were correct, this would explain not only its effect on patients’ lungs but also the prevalence of a wide range of symptoms related to clotting and bleeding that clinicians were noting in acute cases of COVID-19. The group published various papers in 2020 to show clotting pathology in the acute phase, including a significant microclot load as viewed under a fluorescence microscope. In 2021, Pretorius turned her attention to those patients who had recovered from the acute phase but still suffered from lingering, debilitating symptoms that were not present before they were infected.
The first breakthrough in recognising that long COVID could be a valid medical condition occurred when Pretorius, again using fluorescent microscopy, found significant microclot formation in the blood samples from a small group of patients suffering from long COVID. Curious about the content of these insoluble microclots, she approached the proteomics laboratory at SU’s Tygerberg campus to study their protein content. Working with the senior proteomics specialist in the Mass Spectrometry Unit at SU’s Central Analytical Facilities, Dr Maré Vlok, they found an overload of inflammatory molecules ‘trapped’ inside the microclots.
“The trapped molecules contained clotting proteins such as fibrinogen, as well as alpha-2 antiplasmin. We immediately realised that the presence of these proteins would significantly inhibit the body’s ability to break down the clots,” she explains.
Alpha-2 antiplasmin is a molecule that prevents the breakdown of blood clots, while fibrinogen is the main clotting protein. Under normal conditions, the body’s plasmin-antiplasmin system maintains a fine balance between blood clotting (the process by which blood thickens and coagulates to prevent blood loss after an injury) and fibrinolysis (the process of breaking down the fibrin in the coagulated blood to prevent blood clots from forming).
The findings of this study were published in the journal Cardiovascular Diabetology in August 2021.
Current thinking is that these insoluble microclots inhibit or even temporarily block blood flow to capillaries and subsequent oxygen transfer to tissue. The lack of oxygen in various parts of the body can account for most of the symptoms of long COVID. This theory was supported by Pretorius and her team’s discovery that the Omicron variant caused significantly less blood clotting than the Beta and Delta variants and, therefore, less severe COVID-19 symptoms.
The replication problem
In the natural sciences, one of the most important indicators of the validity of your findings is the extent to which others obtain the same results, many times over, when replicating your work. But what happens when there is no widely available protocol for general practitioners or specialists to detect the microclots in circulation, which would confirm Pretorius’ findings?
To address this problem, Pretorius set out to raise the necessary funds to duplicate the 2021 proteomics study on a much larger scale, this time working with blood samples from a cohort of 99 long COVID patients and a control group of 30 healthy individuals (instead of only 15 as in the 2021 study).
This larger proteomics study was funded by the Long COVID Research Charitable Trust, established with a donation from Mr Koos Pretorius from ENSafrica. The crowdfunding platform Kernls and the research foundation PolyBio in the United States enabled the purchase of a flow cytometer for the lab. Also, a donation from the COVID-focused scientific investment and direct gifting fund Balvi funded an automated system that fits onto the fluorescence microscope for studying platelet and microclot pathology.
Proteomic analysis of blood samples is costly, but it was crucial to testing whether the findings of the 2021 study were indeed the rule and not an exception.
“Proteomics is the study of proteins, also called ‘the building blocks of life’. Human and animal cells contain thousands of proteins with highly complex and diverse functions, from regulating development and reproduction to warning the immune system against invaders. Investigating how the body’s immune system reacts is therefore crucial to understanding how the coronavirus disease develops,” Pretorius explains.
It took 8 months to perform the proteomic analysis and analyse the nearly 130 samples. According to Dr Maré Vlok, this is the largest proteomic analysis he has ever performed.
The results, published in October 2022 in the journal Cardiovascular Diabetology, again confirmed the presence of a significant number of large molecules known to significantly reduce the body’s ability to regulate clot formation (coagulation), trapped in microclots in the blood samples from long COVID patients.
According to Pretorius, research groups from all over the world have recently begun to confirm their findings. This includes evidence of the increased presence of clotting proteins such as the von Willebrand factor (a prothrombotic molecule that favours clot formation) and alpha-2 antiplasmin (which inhibits the breakdown of clots) inside microclots in the blood of patients with long COVID.
Importantly, researchers have also found a significant number of previously unidentified antibodies and autoantibodies that might be associated with or trapped inside the microclots.
‘The clot thickens’
According to Pretorius, their study was the first to identify both antibodies and autoantibodies trapped, specifically, inside the microclots in the blood samples of patients with long COVID. The results are in line with other research groups’ identification of antibodies and support the GAO’s identification of the body’s autoimmune response as one of the causes of long COVID.
Dr Arneaux Kruger, a medical doctor pursuing an MSc under Pretorius’ guidance and first author of the aforementioned article in Cardiovascular Diabetology, says further research is needed to fully understand the significance of this finding: “The antibodies could have been formed in response to the infection, but they could also potentially be autoantibodies formed against the host itself or against the microclots we identified. In other words, it could be that, in people with long COVID, the immune system is trapped in a vicious cycle as it continues to produce autoantibodies in response to the antibodies trapped in the microclots.”
The implications of this finding, as understood thus far, are far-reaching and confirm what Pretorius has discovered in over a decade of research on the link between dormant microbes and chronic inflammatory diseases such as Parkinson’s, Alzheimer’s and, more recently, the debilitating, poorly understood medical condition myalgic encephalomyelitis or ‘chronic fatigue syndrome’ (ME/CFS).
As far back as 1996, the systems biologist Prof Douglas Kell from the University of Liverpool predicted that more and more disease states would be found to have a microbial origin. In 2011, after reading each other’s work, Pretorius and Kell started collaborating. In 2015, at a time when the general understanding was that blood in healthy organisms is a sterile environment, they coauthored a review paper in FEMS Microbiology Reviews titled ‘The dormant blood microbiome in chronic, inflammatory diseases’. The two researchers have been collaborating ever since and are currently applying decades of knowledge and expertise in their attempts to make sense of long COVID, as it develops in real time.
In a recent webinar, Dr Mark Walsh, lead emergency medicine physician at the St. Joseph Regional Medical Center in Lewiston in the United States, stated that the foundational work by Pretorius, Kell and Laubscher has helped his team explain the clotting complications in patients with acute COVID-19: “We could not understand why patients would clot and bleed at the same time. We now have the pathophysiological foundation for a point-of-care bedside medicine approach, based on the foundations of excellent research.”
While the COVID pandemic has brought many dark clouds, there may be a silver lining: This renewed focus on post-viral diseases will bring relief to millions of people who have been silently suffering from ME/CFS and other chronic inflammatory diseases.
Bringing in the big guns: machine learning and genomics
Pretorius is also part of a multidisciplinary group of machine learning, physiology and genomics experts that will investigate the risk factors for developing complications after receiving the COVID-19 vaccine. This project is funded by a R3,3 million grant from the South African Medical Research Council.
“Although vaccination is our only hope to return to any form of normality, there are certain individuals that may suffer from adverse effects due to their genetic makeup or a general chronic inflammatory status, making them more prone to clotting pathologies and even death,” Pretorius explains. This means that, after vaccination, some individuals may develop clotting pathologies even if they never had acute COVID-19.
“There is an urgent need to determine the overall risk factors for developing complications,” says Prof Bruce Watson, principal investigator and holder of the Capitec Chair in Applied Artificial Intelligence at SU. The current understanding is that the origin of these risk factors is genetic and/or environmental, or related to the manner in which proteins are produced in the body.
Going forward, Pretorius and Dr Chantelle Venter from SU’s Department of Physiological Sciences will be looking for clotting pathologies in the blood samples of healthy individuals who experienced complications after vaccination (‘vaccination injury’), and in samples from individuals suffering from long COVID after acute COVID-19 infection, and from vaccination injury. The control group will consist of healthy, vaccinated individuals who have either never had acute COVID-19 infection or have fully recovered.
Prof Maritha Kotze from SU’s Department of Chemical Pathology will determine common genetic risk factors associated with inherited thrombophilia (a condition in which your body tends to form blood clots).
Watson’s team will make use of the latest machine learning methods to analyse associations between the various patient characteristics.
“Our approach is to build a dataset that includes patient characteristics such as their genetic, proteomic and environmental data, and their comorbidities. Using relatively sophisticated but well-studied machine learning methods, we will cluster participants to identify common features among unique clusters,” he explains.
They will then use supervised learning methods to determine the relationship between blood markers, genetic and environmental factors, and those patients in the dataset with post-vaccine malaise. By taking these relationships into account, the team should be able to rank combinations of risk factors according to their impact on post-vaccine health.
The results of the study may finally be converted into clinical guidelines for both pre- and post-vaccine patients.
The way forward
Pretorius and her team’s microclot work is being replicated across the globe by teams such as the one at Dr Beate Jaeger’s Mülheim Clinic in Germany and, in the United Kingdom, at the University of Sheffield Hallam and the University of Manchester. Also, the Balvi research foundation has funded a microclot detection system for Kell’s laboratory at the University of Liverpool. In the United States, Pretorius will assist Prof David Putrino, Director of Rehabilitation Innovation for the Mount Sinai Health System, in establishing the microclot method in his laboratory.
“There is still a lot to learn about the pathophysiology of long COVID,” Pretorius concludes. It has taken 3,7 billion years for proteins to evolve into this complex immune system that we have today – no surprise then that we are still in the starting blocks of truly understanding it."
Useful links
SA research shedding light on role of microclots in long COVID
COVID-associated coagulopathy: The clot thickens (or not?)
The role of tiny blood clots in long COVID
Could microclots help explain the mystery of long COVID?
First evidence of inflammatory microclots in blood of individuals suffering from long COVID
Interview with Dr Resia Pretorius: Long COVID microclots
Twitter: @MatiesResearch