The future of microbiome-based therapeutics
Wiida Basson
Illustration by Roulé le Roux
A new study on the relationship between our gut microbiome and the brain provides a stepping stone for future research into microbiome-related therapeutics to prevent or treat mental health disorders.
Bacteria have been around for the past 1,6 billion years and co-evolved with humans to perform a range of beneficial functions in our bodies: They help digest our food, regulate our immune system, protect against other disease-causing bacteria, and produce vitamins.
But the more we discover about the human gut microbiome, the more questions arise about the influence that trillions of these microorganisms may also have on our mental condition, mood, and overall psychiatric well-being.
The known genes of the microorganisms in the human microbiome outnumber human genes by a hundred to one, and most of them have not even been identified yet. So, while we may be aware of the intricate symbiosis between ourselves and the microorganisms helping us to thrive (or not), there is still much left to unravel.

Prof Leon Dicks at work in his laboratory | Photo by Stefan Els
A gut feeling for research
For the past 35 years, Prof Leon Dicks has dedicated his research in the Department of Microbiology at Stellenbosch University to the study of lactic acid bacteria, a group of bacteria that are beneficial to humans.
He has focused specifically on their probiotic properties and ability to produce antimicrobial peptides under certain conditions. This research has led to the development of, amongst other patents, the probiotic EntiroTM, produced and marketed by Cipla Medpro.
Despite these advances, Dicks strongly believes that we are yet to realise the full potential of the human microbiome. “I am two years from retirement, but the field of microbiology is only now coming into full bloom. We are standing on the cusp of major new discoveries in the field of microbiology in general, and specifically in the emerging field of a microbial-based approach to the treatment and management of psychiatric disorders and serious diseases such as cancer,” he explains.
In January 2022, Dicks embarked on a second doctoral dissertation to better understand the connection between the gut and the brain. The result, for which he was awarded a DSc degree in March 2023, is a powerful synthesis of the current knowledge in this emerging field, and our understanding thereof. Dicks has made this research accessible by consolidating several very complex ideas regarding the gut microbiome and the gut-brain axis into a coherent story.
Our second brain
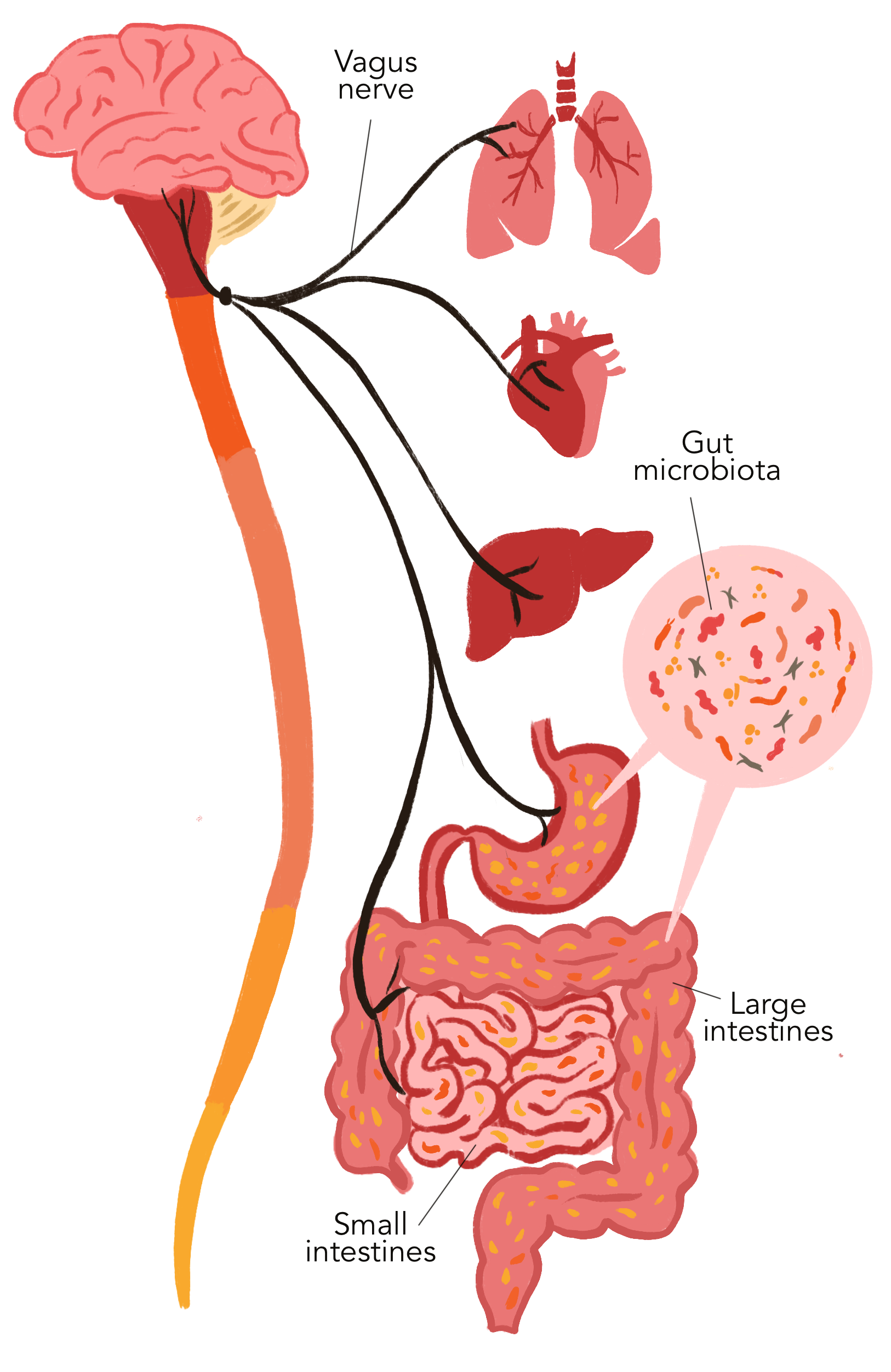
In discussing the research for his dissertation, Dicks firstly highlights the importance of our "second brain" — a community of almost 5 000 microbial species living in the human gut, of which 90% are bacteria. This "brain within the gut" sends out signals to our central nervous system and reacts on signals received from the brain.
“We know a lot of the microbes in the human gut, but there are many more that we have not yet identified. We also know that they are talking to each other by means of chemical signals, a process we call ‘quorum sensing’. These bacteria are having conversations with each other, and it may sound like a lekker kuier [a pleasant social event], but it’s actually a biochemical process that has an effect on our psyche,” he explains.
Most of the time, this intricate control system of chemical signalling and immune response keeps the gut microbiome in a balanced state. A healthy gastrointestinal tract is characterised by such a balanced gut microbiome with a core population of beneficial microbiota. When things go wrong, however, changes in the microbial population will ultimately affect communication with the central nervous system, and vice versa.
But exactly how does this two-way communication process take place?
The highway of communication is the vagus nerve, which runs from your brain to your large intestine and the enteric nervous system. Importantly, fibres of the vagus nerve are not in direct contact with the gut or intestinal microbiota. Instead, signals reach the gut microbiota via 100 to 500 million neurons from the enteric nervous system. These neurons form a network of nerve fibres in the muscular layers of the digestive tract.
According to Dicks, it is evident that the intestinal barrier is controlled by fine-tuned communication between gut microbes and the host immune system. Understanding this communication, however, is another matter completely.
The complexity of these interactions raises questions around our current level of understanding, and explains why it has been so difficult, up to now, to develop specific therapeutic targets, Dicks writes in the conclusion to his dissertation.

Research in the laboratory of Prof Leon Dicks focuses on the identification and classification of lactic acid bacteria isolated from humans, animals, plants, and the environment. The scientists involved are interested in the antimicrobial peptides produced by these bacteria, which could be developed as an antibiotic or probiotic. | Photo by Stefan Els
Linking neurotransmitters and bacteria
Take the case of serotonin as an example, he says. Serotonin is one of the key neurotransmitters in the brain, regulating our appetite, gut motility, mood, cognition, and sleep patterns. As much as 80% of this important neurotransmitter is produced in the gastrointestinal tract by, for example, E.coli, Hafnia, Bacteriodes, Streptococcus, Bifidobacterium, Lactococcus, Lactobacillus, and Morganella.
“Serotonin production by gut microbiota may have a greater effect on the central nervous system than originally anticipated,” he writes in one of his published dissertation papers. A study on germ-free mice, for example, has shown that neuronal dysfunction could be reversed by the recolonisation of specific gut microbiota, especially those that produce short-chain fatty acids.
These acids, which stimulate endothelial cells to produce serotonin, are largely produced in the colon by Bifidobacterium, Lactobacillus, Lachnospiraceae, Blautia, Coprococcus, Roseburia, and Faecalibacterium.
In humans, changes in serotonin levels are also associated with irritable bowel syndrome and, in patients with ulcerative colitis and Crohn’s disease, drastic increases in serotonin-immunoreactive cells in the colon. Furthermore, a lack of communication between gut microbiota and the enteric nervous system has been directly linked to dysbiosis (an imbalance in your gut microbiota) and other gastrointestinal disorders.
A recent report suggests that certain neurotransmitters may even serve as growth substrates for intestinal bacteria. In other words, without these neurotransmitters, some bacteria would be incapable of surviving in the human gut. This observation raises questions about a possible symbiotic relationship between bacteria and neurotransmitters.

Looking into the future
Dicks is convinced that, in the not-so-distant future, the gut microbiome will be integral to the development of novel therapeutics, probiotics, and psychobiotics aimed at treating gastrointestinal disorders, improving cognitive functions, and preventing or treating mental disorders such as depression and schizophrenia, as well as conditions on the autism spectrum.
While we know that gut microbiota have an immense impact on the gut-brain axis and on overall mental health, Dicks warns that our understanding of exactly how gut microorganisms affect cognitive behaviour, mood, and neuropsychiatric disorders remains limited.
Perhaps it is not time for retirement just yet — there is simply still too much to learn: “The Creator keeps us busy!” he quips with a glint in the eye.
What are probiotics?
The International Scientific Association for Probiotics and Prebiotics defines "probiotics" as live microorganisms that confer a health benefit on the host upon administration.
What are microbiota?
The term "microbiota" refers to all living microorganisms — including bacteria, archaea, fungi, and algae — that live in a particular environment, such as the human gut.
What is a microbiome?
The term "microbiome" refers to more than just a collection of microbiota. Any given microbiome has its own distinct properties, functionalities, and interactions with its environment. This is why the human gut microbiome is referred to as ‘the second brain’.

The research initiatives reported on above are geared towards addressing the United Nations’ Sustainable Development Goals number 3 and goal number 2 of the African Union’s Agenda 2063.
Useful links
SU’s Department of Microbiology
Twitter: @sciencesun; @MatiesResearch and @StellenboschUni