A spice as medicine: The possible role of turmeric in treating Parkinson’s disease
Engela Duvenage
Illustration by Ronel van Heerden
In the past, Delia (a pseudonym) often used turmeric to provide colour and taste to rice, bobotie, and curries. Recently, however, she’s also started adding a sprinkle of this yellow spice to fruit smoothies, milkshakes, and even coffee — in which case the flavour isn’t always complementary. Fortunately, her homebrew’s strong aroma helps to mask the bitter, mildly earthy taste of the spice.
Delia always gulps her drink down quickly. After all, she isn’t drinking turmeric for the culinary experience. She’s doing it for her brain.
She recently read that the polyphenol curcumin found in turmeric has strong antioxidant and anti-inflammatory properties and that, in India, a paste made from the yellow spice is commonly applied to wounds. Moreover, she learnt that researchers are investigating its possible role in guarding against and treating Parkinson’s disease (PD).
The burden of disease
Members of Delia’s extended family have suffered from PD, a degenerative neurological condition that develops when brain cells stop producing enough dopamine, a brain chemical that coordinates movement.
This so-called "movement disorder" is characterised by, among other symptoms, rigidity, resting tremors, sleep disturbances, and depression.
Delia had to watch her grandfather (73) fade away from a headmaster who could mesmerise a school assembly to a frail old man who took ages to respond to questions, and then only in the softest of voices. Often, his hands shook so uncontrollably that he couldn’t drink coffee without spilling. At other times, he would freeze to the spot, unable to shuffle down the passage to make it to the bathroom in time.
The experts called his increasingly slow, halting movements "bradykinesia". This, along with the tremors, is a typical symptom of PD, a disease for which there is still no cure. The only known therapy, the amino acid (or protein "building block") levodopa, is merely palliative and only temporarily enhances levels of the neurotransmitter dopamine in malfunctioning brainstems.
The effectiveness of levodopa wears off over the course of a day, and often over a patient’s lifetime. It also comes with side effects such as lightheadedness, a loss of appetite, and even hallucinations. For some patients, the dyskinesias — the involuntary erratic writhing of one’s face, trunk, or limbs — are worse than the PD symptoms themselves.
These symptoms start unobtrusively. A loss of taste is often the first sign of emerging PD, many years before symptoms such as a masked, unemotional face, tremors, or forgetfulness make their presence known in full colour.
Delia’s husband sometimes chides her when she pulls a face while drinking her turmeric-infused coffee: “Why do you torture yourself like that? How do you even know it’s going to help?”
“I don’t. Yet. But how do you know it’s not going to help? I’m not taking any chances. It’s impossible to reverse brain damage. I’m willing to try anything to ensure that I don’t get PD. I don’t want to end up like Grandpa Dan,” she’ll shoot back, remembering the toll that his constant care, especially towards the end of his life, took on his immediate family and carers.

Abigail Braun (MSc student, on the left) and Prof Soraya Bardien, using high-resolution melt analysis to identify individuals who carry sequence variants that are known to cause Parkinson’s disease. | Photo by Wilma Stassen

Lusanda Madula (BScHons student, on the left) and Kathryn Step (PhD student) are analysing genetic data from South Africans living with Parkinson’s disease to identify novel disease-causing sequence variants. | Photo by Wilma Stassen
Possible new therapy
Over the past decade, research groups from countries such as India, Pakistan, Italy, Mexico, Iran, and China have explored the use of curcumin as a pharmacotherapy to stave off PD, and possibly even cure the neurological damage it has already caused in patients who suffer from it.
These groups are motivated in part by existing knowledge of curcumin’s purported healing benefits as a strong antioxidant and anti-inflammatory, and its wound-healing abilities. Some people use it to detox their liver and gallbladder, guard against blood clotting, or improve their gut flora and iron levels.
Recently, in Stellenbosch University’s (SU’s) Faculty of Medicine and Health Sciences, members of its Parkinson’s Disease Research Group have also started studying curcumin’s use in PD treatment, and its role in gut-brain interaction.
This research is funded by the National Research Foundation and the South African Medical Research Council.
“Our studies are still in their very early stages, with tests so far only being done on cell lines and not even animal models yet,” says Prof Soraya Bardien, head of the Parkinson’s Disease Research Group in the Division of Molecular Biology and Human Genetics at SU’s newly launched Biomedical Research Institute (BMRI).

Jodi Dovale (BScHons student, on the left), Jessica Burns (PhD student, in front) and Amy Buck (PhD student), examining cells that will be treated with curcumin-encapsulated nanoparticles. | Photo by Wilma Stassen
Finding the root cause
Bardien says there are many theories about how and where exactly in the body PD starts. The strongest of these maintains that it’s in either the gut or the brain.
In a 2021 review paper published in the European Journal of Neuroscience, Bardien’s team explains that a pathological hallmark of PD is the buildup of toxic forms of presynaptic alpha-synuclein, a neural protein, in specific structures in a patient’s gastrointestinal tract and brain called "Lewy bodies". This might be because the protein moves along the vagus nerve that connects the brain to the gut.
With time, as more such Lewy bodies accumulate, patients start experiencing problems with thinking, movement, behaviour, and mood.
The starting point for the movement of alpha-synuclein seems to vary from patient to patient. This could explain why people with PD experience different symptoms and clinical versions of the disease. Some often experience gastrointestinal problems, for example, while others do not.
Evidence is still scant on whether surgical alterations of the vagus nerve or operations to remove gut-associated lymphoid tissues, such as the appendix and tonsils, provide protection against PD.
Curcumin as toxin magnet
Researchers are interested in testing whether curcumin can bind to specific parts of alpha-synuclein, specifically its non-amyloid-beta component. The idea is that, if curcumin can work like a "magnet" to attract and then bind with alpha-synuclein, the molecules will be quickly excreted from the body in tandem. Ultimately, this will prevent the protein from building up in the body to the point of toxicity.
A drawback is that curcumin is excreted quickly, and in great quantities. This makes it difficult for the body to take up enough of this polyphenol for it to be of any real value, and for it to cross the brain-blood barrier and target the brain cells that are lost in disorders like PD, Bardien explains. Even if proven helpful, it is likely that curcumin will have to be taken as a lifelong complementary supplement.
Two of Bardien’s PhD students, Jessica Burns and Amy Buck, are investigating whether it is possible, and helpful, to cover curcumin granules with nanoparticles made from organic and biodegradable materials. (Nanoparticles are very tiny structures, typically less than 100 nanometres in diameter, that can be generated by natural processes or artificially made.)
The point of their work is to determine whether this technique will help slow down the excretion of curcumin, improve its crossing of the blood-brain barrier, or both — thereby boosting its ability to provide neuroprotection to people living with PD.
Burns says their studies are motivated by others that have shown the use of nanoparticle-encapsulated curcumin to provide better results in the treatment of diseases. They are currently working with commercially acquired neuroblastoma cell lines, but hope to also do tests on the cells of people with genetic forms of the disease.
Burns and Buck’s studies focus specifically on the mitochondria of brain cells, which they believe play an integral role in the development of PD.
“The mitochondria are the body’s energy producers. They are like the body’s ESKOM. If the mitochondria shut down, the body shuts down too,” explains Bardien.
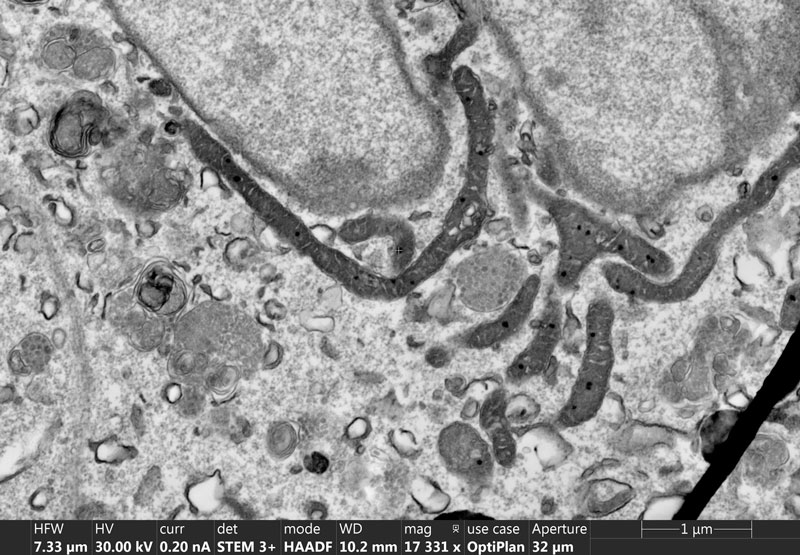
Scanning electron microscopy images of cells enable researchers to visualise the morphology of tiny intracellular structures, such as the mitochondria. | Image courtesy of SU’s Central Analytical Facilities
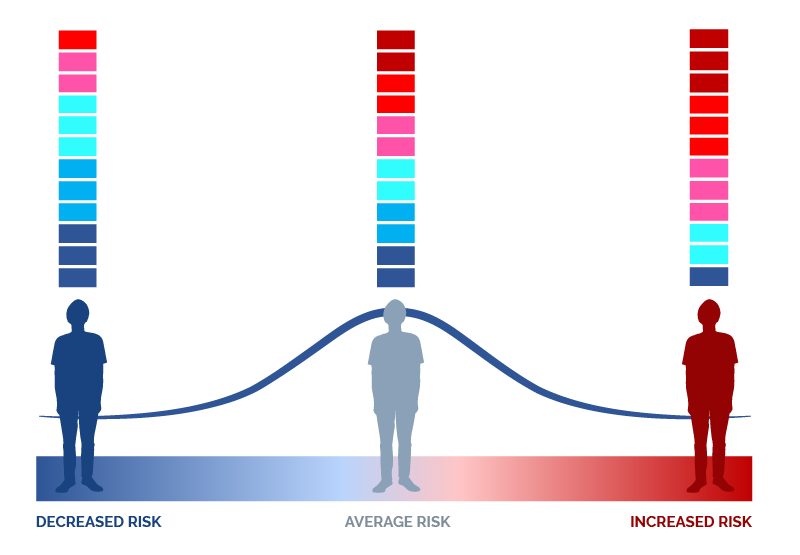
An overview of the principle behind polygenic risk score (PRS) calculations. Genetic variants can be associated with an increased or decreased risk of disease. To calculate an individual’s PRS, their increased or decreased disease risk variants are added together to provide information on their personal risk for disease development, compared to that of other individuals.
Could genetic factors be at play?
PD is the fastest-growing neurological disorder in the world. This is likely also the case in Africa, although this remains to be shown.
Patients worldwide receive the same treatment — the drug levodopa — but their treatment is dependent on a diagnosis. In countries that have more neurologists at hand, this is easier to receive than in others. Moreover, treatment of PD in parts of sub-Saharan Africa (excluding South Africa) is made difficult by a lack of drugs.
The environmental and genetic factors possibly linked to the disease have been studied widely in various populations across the globe, but similar investigations among African populations are still few and far between. In point of fact, the Parkinson’s Disease Research Group at SU is the only one of its kind on the continent.
Established in 2000 by neurologist and movement disorder specialist Prof Jonathan Carr, this multidisciplinary group studies the genetic basis of and molecular mechanisms at play in PD development.
“Our major research interest is trying to find out the cause of Parkinson’s. It is not just intellectually important but an absolutely crucial step to finding a cure,” Carr wrote in 2012 in a South African newspaper.
“It’s a complicated business, and the truth is that by 2023 we still don’t really know the cause of Parkinson’s disease,” he said a decade later in a video released on Parkinson’s Disease Awareness Day on 11 April 2023.
Bardien agrees with this sentiment. “Despite having been discovered in Western medicine some 200 years ago, the exact causes of PD are still not quite known. An interplay between environmental factors and genetic causes is most possibly at its root.
“So far, genetic factors have only been linked to 10 to 15% of all cases of PD, and are often associated with families with a history of early-onset PD,” she notes.
Patients with early-onset genetics-related PD are often diagnosed in their twenties or early thirties, whereas others tend to receive their diagnosis around retirement age only.
One of the important findings from the work of Bardien’s research group so far is that there may be a founder effect for PD in some Afrikaner families, dating back to the 1660s. The term "founder effect" refers to the reduction in genetic variation that occurs when a new population is founded by a very small subset of a large population. This often increases the occurrence of otherwise rare inheritable diseases in the new group.
Also, as part of an international collaboration, neurologist Dr Riaan van Coller was able to identify a novel pathogenic variant in the PTRHD1 gene in a specific Xhosa family with a history of early-onset PD and intellectual disability.
These findings are the result of studying DNA samples gathered since 2006 as part of the South African Parkinson’s Disease Research Collection database, housed at SU. Collecting the 687 samples from volunteers was a mammoth task, and saw research nurse Sr Debbie Acker, now the project manager at FAMCRU (SU’s Family Centre for Research with Ubuntu), travel across the country to do so.
Most of the work on these samples has been concerned with specific single-gene variations previously identified by other researchers as being linked to the development of PD.
“By looking at these single genes that others have identified, we’ve found that the pick-up rate in the South African population is very low. That means that we are not finding the same pathological gene variations in families with two or three generations of PD as we do elsewhere in the world, for instance in America or Europe. It makes me think that if we have a pathogenic variant in these families, it’s in a yet undiscovered gene,” Bardien notes.
She considers studies on the genetic differences between Southern African populations to be of great importance.
“We know the genetics of populations in sub-Saharan Africa to be the most diverse globally. Because the genetics of PD in these populations has not been well studied, it is vital that these populations be investigated to determine whether they have novel genetic causes of this disease.”

Prof Soraya Bardien, head of the Parkinson’s Disease Research Group | Photo by Wilma Stassen
The GP2 as research booster
“Funding in South Africa for PD research is very limited, given all the infectious and cardiovascular diseases we have. We’ve never been able to do sequencing of the whole human genome, in search of possible new variations specific to our unique South African population,” Bardien explains.
As such, she is excited about her research group’s involvement in the Global Parkinson’s Genetics Program (GP2), and what it could mean for the study of the genetics of PD in African populations. Funded by the Michael J. Fox Foundation, the GP2 is an ambitious initiative to genotype 150 000 volunteers around the world to better understand the genetic architecture of PD. According to the GP2 website, there is still much to learn about genetic risk factors, something that can only be achieved by “working collaboratively and openly sharing data, processes, and results”.
Bardien says the endeavour, which provides free whole-genome genotyping and sequencing to participating countries, is a “game changer” and already includes 100 research teams from around the world.
“The idea is to do genotyping in underrepresented populations in especially Africa, Asia, South America, and pockets of Europe where such research cannot be done, to help them recruit study participants, and to do whole-genome sequencing for free if the necessary genetic labs aren’t locally available,” she explains.
“To my knowledge, this has not been done for any other disease at this scale before.”
Identifying and characterising carriers of specific genetic variations involved in the development of PD is “imperative in light of related gene-targeted therapies increasingly being developed”, Carr and 106 other researchers wrote about GP2 in a 2023 paper published in the journal Movement Disorders.
Data for Africa
Bardien is excited about what SU’s involvement in GP2 could mean for local and global PD science.
“We might get some real answers about the genetics of South African people with PD. We might even find, through this consortium, that there is a bigger genetic component worldwide to PD than the 10 to 15% of cases that we currently know of.”
“Sequencing is done for free. The data is then used to do global analysis. At the same time, we receive our own data back, which we are allowed to analyse and publish on our own.”
After only a few months, Bardien’s team has already received back their first batch of genotyping and whole-genome sequence data. It is now up to PhD student Kathryn Step and human genetics MSc student Abigail Braun to analyse this data in detail, in search of possible new variants in specific South African individuals and their families.
“Populations in Africa are some of the oldest worldwide, therefore our genetic diversity in the causes of PD could also be the greatest,” Bardien notes. “Already, the consortium has had to adapt some of its protocols to be able to better analyse the ancestral makeup of participants from the South African population.”
Spiced gold
Bright yellow turmeric powder, also known as “Indian saffron” or “the golden spice”, is made from the ground roots of a rhizomatous, herbaceous perennial native to tropical South Asia. Apart from being a major ingredient in curry powder, it is also used as a dye. Curcumin, one of the components of turmeric, has powerful antioxidant and anti-inflammatory properties.Did you know?
- Parkinson’s disease is a brain disorder that affects 7 to 10 million people worldwide, most of them men.
- The disease is vastly understudied in Africa.
- Most people who develop Parkinson’s are over 60, but one in ten are under 50 years of age.
- The Global Burden of Disease Study 2015 estimated that there may be nearly 13 million people with Parkinson’s by 2040.
- Parkinson’s Awareness Day is observed on 11 April.
“Parkinson’s is a real disease. It affects at least 3% of the population worldwide. There are many people suffering from it in South Africa, but it’s not always properly diagnosed. It’s a horrible disease. People who have it are often not understood, possibly because of the movements that go along with it. They suffer from depression and apathy. It’s often not seen as a disease. Some people think it’s witchcraft, or something else. They think people can snap out of it. We really need to raise awareness about the disease.”
— Prof Soraya Bardien, head of the Parkinson’s Disease Research Group in the Biomedical Research Institute at SU
Video by SpotlightYOPD

The research initiatives reported on above are geared towards addressing the United Nations’ Sustainable Development Goals number 3 and goal number 3 of the African Union’s Agenda 2063.
Useful links
The Michael J. Fox Foundation for Parkinson’s Research
The Genetic Epidemiology of Parkinson’s Disease consortium
Mayo Clinic on Parkinson’s disease
GCNetwork, facilitator of genetic counselling services in South Africa
Parkinson's. Not just an old person's disease
Twitter: @MatiesResearch: @StellenboschUni; @SUhealthsci