The interconnected, growing threat of TB in animals and humans
Jorisna Bonthuys

Prof Michele Miller photographed with an immobilised African elephant in the Kruger National Park | Photo by Phillip du Plessis
Back in 2016, the death of a certain African elephant bull in the Kruger National Park (KNP) left researchers puzzled.
Rangers found the animal’s carcass near the Tshokwane camp in the southern part of the park. The elephant, estimated to be approximately 45 years old, had no external wounds or visible injuries, but its condition was poor.
Initially, scientists suspected the cause of death to have been bovine tuberculosis (TB), caused by Mycobacterium bovis. Bovine or animal TB is a significant, infectious disease affecting livestock and wildlife populations. M. bovis was diagnosed in the park in 1990 and is now endemic to this conservation area.
But this case was different. Genomic sequencing and analysis showed that the elephant had suffered from human TB caused by Mycobacterium tuberculosis, which is transmissible from humans to animals. This is the same TB bacteria that cause serious illness in humans worldwide and affect predominantly the lungs.
This was the first time researchers had identified a fatal case of human TB in a free-ranging African elephant.
The finding suggested that M. tuberculosis could be a greater threat to wildlife populations in Africa than previously realised.
“We were puzzled by this,” recalls Prof Michele Miller. “How is it possible for wild animals to get infected with human TB in the KNP?” Miller, who heads up the Animal TB Research Group at Stellenbosch University (SU), holds the DSI-NRF South African Research Chair (SARChI) in Animal TB.
To answer this and other questions, Miller and her team are investigating bovine TB in the KNP and other conservation areas, including the Hluhluwe–iMfolozi Park in KwaZulu-Natal. They are also doing research in rural communities living near or adjacent to protected areas where bovine TB has been documented.
The researchers, based in SU’s Division of Molecular Biology and Human Genetics in the Faculty of Medicine and Health Sciences, are determined to understand exactly how the transmission of TB occurs between wildlife, livestock, and people.
One of their research endeavours, for instance, is an elephant surveillance programme that involves screening the KNP’s elephants for mycobacteria. Since 2016, no more cases of human TB in elephants have been detected. Five elephants have, however, been diagnosed with animal TB.
A multi-host disease of concern
“TB is often thought of as a disease that affects only humans when, in fact, it is a multi-host disease [that affects many species],” Miller points out.
Also, there is not simply a single pathogen at work — several of the different species of mycobacteria that belong to the TB complex could be responsible for causing the disease in animals.
Every case of TB is caused by related bacteria within the Mycobacteria tuberculosis complex (MTBC). These pathogens include M. tuberculosis (the causative agent of human TB) and M. bovis (which causes bovine TB).
Most mammal species can be infected with bacteria in the MTBC complex, M. bovis being the primary cause of TB in wildlife and livestock.
Currently, 25 wildlife species in South Africa — including rhinos, cheetahs, lions, and buffalo — are known to be affected by animal TB.
This chronic disease has also been increasingly detected in new species and at a seemingly high prevalence rate among buffalo, wild dogs, lions, and warthogs. This can have far-reaching consequences for conservation, the livestock industry, and public health.
Miller says the KNP offers “a real-world” example of how introduced TB bacteria can impact free-ranging populations of wildlife that interact with domestic animals and humans.
“It is a global problem, however,” Miller says. “There are literally tens of thousands of TB-infected animals all over the world.”
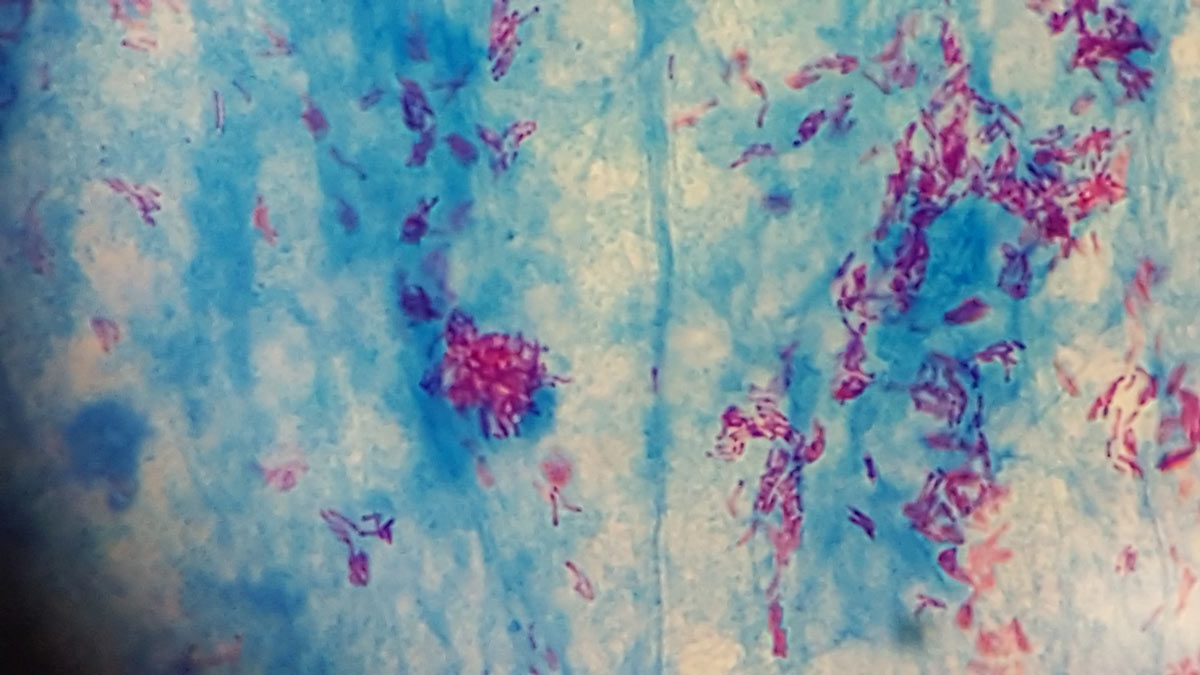
Photo showing a Ziehl-Neelsen (acid-fast-stain) positive (pink) mycobacteria in smear from elephant tissue infected with Mycobacterium tuberculosis | Photo by Lin-Mari De Klerk-Lorist
Old problem, new challenges
It is believed that bovine TB was brought to South Africa by colonial settlers importing infected cattle in the 19th century. “Primarily, it has been a disease among domestic cattle,” Miller says. “It is only since the 1990s that we’ve really been aware that it can spill over from domestic animals into wildlife in the KNP.”
Centuries ago, as high as 20% of human cases of TB were caused by transmission from cattle, primarily via unpasteurised milk. This is one of the reasons why TB is, today, an actively controlled disease among animals. “Although surveillance and monitoring programmes have reduced TB prevalence in cattle, animal TB is rearing its head again,” Miller says.
Several factors determine whether a population can maintain and spread animal TB infection. These include the presence of enough susceptible hosts, how long the bacterium infects its hosts without killing it (often dependent on the status of the host animal’s immune system), and whether the bacteria can remain viable long enough to find new hosts.
Although animals are typically infected with M. bovis and humans with M. tuberculosis, reports are increasingly noting reverse infections from animals to humans and vice versa, Miller says. “There is a gap in our knowledge as to what’s leading to these infections jumping between species.
“What we are seeing is that in a wildlife park, M. bovis crosses the border from buffalo herds to goats and cattle, to soil and water, and subsequently to humans," she says. “People with a high TB burden who, for example, care for captive elephants can also spread human TB to these animals.”
An artist’s representation of animal TB transmission in North America and Europe and in Africa | Illustrations by Laura Donohue
Route(s) of transmission
In a multiple-host system such as the KNP, there are many routes for transmitting TB among or between wildlife species.
Some species are considered important “reservoir” hosts of TB, which means they maintain the infection within their population and spread the disease to other species. While not all of these reservoir hosts, such as buffalo and greater kudu, exhibit signs of disease, get sick or perish, the existence of many of them ensures that infections persist within the park’s ecosystem. On the other hand, “accidental” or “spillover” hosts typically contract TB through contact with a reservoir host.
Indirect spread may occur through contact with the mycobacteria in the saliva, nasal mucus, faeces, or urine of infected animals. This method of transmission usually occurs through close contact between individual animals, particularly in social species like African buffalo.
Moreover, M. bovis in animals’ respiratory secretions can contaminate the environment since the pathogen is able to survive in moist soil in water holes and mud wallows. This may lead to its transfer between buffalo or to other species. (The prevalence of animal TB in buffalo is estimated to range up to 40% in some areas of the KNP).
Although the mechanisms of transmission between herbivores such as antelope and rhinos are still poorly understood, they also relate to indirect interaction through the sharing of resources such as contaminated water holes and to the consumption of infected vegetation.
Carnivores and scavengers, on the other hand, can be infected by eating infected prey. In addition, African lions have been found to transmit M. bovis through biting, causing infection in other lions.
Recent evidence also suggests that lions and African wild dogs are shedding M. bovis in airborne particles through their respiratory secretions, Miller says.
Wild animals kept captive in zoos, on game farms, and on wildlife ranches can also contract the disease. These infections can be caused by transmission within or between species due to the presence of infected animals or their human caretakers.
Together, these and other transmission pathways enable TB bacteria to survive and spread among wildlife species.
“We are finding that TB bacteria are really, really hardy,” Miller says. “They can remain alive in soil or around water holes for days to months, depending on the conditions.” (Mycobacteria possess a unique, thick lipid cell wall that allows them to not only survive in harsh environmental conditions but also resist the immune responses of their hosts.)
In areas where domestic livestock and wildlife share grazing and water sources, there is a higher risk of spillover (pathogen transmission from animals to humans) and spillback (pathogen transmission from humans to animals) within the wildlife population.
Moreover, certain risk factors such as drought, increased population density, and animals clustering at artificial water and feeding sites can increase the likelihood of bovine TB occurrence in certain species. Interestingly, though, data collected in the KNP indicate that M. bovis-infected white rhinos that receive sufficient food and water appear to be able to contain the disease (that is, suppress the infection, despite the bacteria remaining present in their bodies).

Photo showing the collaborative team of veterinary, capture and airwing staff from SANParks and Stellenbosch University's animal TB researchers required to perform elephant TB surveillance | Photo by Phillip du Plessis
SU researchers leading the pack
SU’s Animal TB Research Group is at the forefront of advances in understanding TB infections in wildlife.
Scientists in the group have reported research showing that the critically endangered African wild dog population in the KNP has a high apparent prevalence of M. bovis infection (82%), with associated mortalities.
In 2022, the group and its research collaborators showed that about one in every seven rhinos in the park was infected with M. bovis. Their findings indicated an estimated prevalence of bovine TB infection of 15,4% across black and white rhino populations.
A recent surveillance study investigating antibodies against M. tuberculosis complex antigens in elephants estimated the level of infection in the KNP’s population to be between 6% and 9%, suggesting that M. bovis and possibly M. tuberculosis infection are more common than previously thought.
The Animal TB Research Group is also part of a global consortium that recently received funding from the European Commission to expand genomics surveillance to include the main pathogens affecting people and animals in South Africa, Mozambique, and Kenya.
This SU-led consortium aims to increase the use of genomic epidemiology to address public health issues such as TB, HIV-1, and antimicrobial resistance in these three countries. The consortium will use a “One Health” approach to conduct early-warning testing in wastewater, and animal surveillance to detect emerging pathogens.
The Animal TB Research Group is leading the consortium’s work on environmental and animal sources of TB. This includes investigating the role of host genetics and immunology in animals’ susceptibility to bovine TB, the genetic diversity of pathogens and their impact on wildlife and livestock, and the development of diagnostic assays for different host species.
One Health approach
South Africa is one of the countries experiencing significant problems around human and animal TB, starting with its diagnosis. SU has a wide portfolio of TB expertise, ranging from that in microbiology, vaccine testing, drug development, and immunology to genetics.
The Animal TB Research Group’s specific focus is the immunology, epidemiology, management, and control of TB in animals, as well as aspects that impact the human-animal interface. The group has adopted the integrated, collaborative, multisectoral, and transdisciplinary One Health approach, which aims to sustainably balance and optimise the health of people, animals, and ecosystems.
Since both animal and human TB can spread across the species boundaries, it is crucial to employ this approach when it comes to detecting, understanding, and controlling TB.
A key area of research in the group is the development of molecular and cellular techniques for detecting infection in animals. This is important, given that bovine TB not only has a severe impact on the health of animals but also has major economic implications.
Many rural communities depend on cattle as a source of meat and milk, and livestock agriculture is the economic lifeblood in many areas. If an infection is detected on a given farm, the farm will be placed under quarantine by agricultural authorities, and the owner will not receive state compensation for the slaughtered animals. This can have a significant impact on livelihoods and food sources, particularly at the household level.
Miller says infected animals in South Africa are slaughtered rather than treated to avoid creating drug-resistant strains of the pathogen. “M. bovis is already naturally resistant to one of the key drugs used for treatment in people.
“The other reason is that we don’t want to keep infected animals that can continue to transmit the disease around. So, generally, when you have a TB control programme for cattle, infected animals are culled to break the cycle of transmission.”

Immobilised buffalo in the Hluhluwe-iMfolozi Park undergoing annual TB testing in KwaZulu-Natal | Photo by Prof Michelle Miller

Photo showing an immobilised African elephant in the Kruger National Park undergoing testing for tuberculosis, using respiratory endoscopy | Photo by Peter Buss
Impacts on conservation
Animal TB not only has a direct impact on livestock, but also holds significant implications for conservation and wildlife ranching.
Since M. bovis is a controlled disease in South Africa, the movement of potentially infected wildlife is restricted. Premises with confirmed cases of infection are typically subjected to quarantine measures.
There are, however, only a few validated tests available for detecting animal TB in wildlife. As a result, the translocation and reintroduction of certain species are impeded.
The discovery of M. bovis infection in the KNP’s rhino population has, for instance, led to a quarantine of rhinos intended for translocation from the KNP to other protected areas.
Miller says infected wildlife are generally not culled unless they are restricted to a small reserve and if management authorities are dealing with the first cases of TB infection in the area. “In some cases, you may want to test and then remove the infected animals, but in a very large system like the KNP where the disease is endemic, it’s not effective or ethical.” In such cases, infected animals are allowed to remain in the system without intervention, unless euthanasia of severely ill animals is called for.
Enabling early detection
Early detection remains key to stopping the spread of bovine TB. It has, however, always been difficult to diagnose this pathogen due to the limited tests available. Species-specific reagents (for rhinos, elephants, and lions, for instance) are not commercially available. “The difficulty is that there aren’t many tests for TB in wildlife species that have been validated and, therefore, are recognised by regulatory officials as proof that those animals should be moved.”
Moreover, conventional mycobacterial culture methods are time-consuming and expensive, with limited sensitivity. As such, more rapid direct methods are needed to detect the presence of TB bacteria, confirm infection, and evaluate the risk of transmission to other animals.
The Animal TB Research Group employs whole-genome sequencing to bypass conventional mycobacterial culture tests. This sequencing technique allows scientists to identify genetic markers associated with TB virulence, host adaptation, and antimicrobial resistance, thereby providing new insights for interventions.
Researchers in the group are also investigating the molecular epidemiology of TB in multiple transmission systems. “That’s an exciting area that we’re going into,” Miller says. “It gives us a much higher resolution to look at transmission among and between species.”
Recent advances in the development of diagnostic tests for M. bovis infection have allowed for the testing of multiple species. This is done by collecting blood and other samples from anaesthetised animals and testing them in SU laboratories for the presence of pathogens.

Members of the Animal Tuberculosis Research Group preparing for field blood collection and TB testing of buffalo in the Hluhluwe-iMfolozi Park | Photo by Prof Michelle Miller

Animal TB researchers working with SANParks veterinary wildlife staff to process field samples in the Kruger National Park. Pictured from left to right: Dr Léanie Kleynhans, Dr Tanya Kerr, Tebogo Manamela and Dr Wynand Goosen | Photo by Prof Michelle Miller
Repurposing existing technology
Researchers in the group have successfully developed various in vitro TB diagnostic immunological blood assays, DNA tests for detecting TB directly, and a novel mycobacterial culturing method for enhancing the growth of bacteria from difficult-to-culture respiratory samples.
The group has been modifying techniques originally used for human TB diagnostics, including a culture-independent rapid polymerase chain reaction (PCR) test used in human TB clinics. “This has been a wonderful piece of technology that can be used as a rapid screening tool across wildlife species,” Miller says. “The technology enables us to run the tests and get the results within a couple of hours.”
SU’s researchers have also considered how existing diagnostic tools for TB can be used in the routine screening of leopards and other wild felids. “We have known for years that lions can get TB,” Miller says. “We have done a lot of research on trying to develop blood-based tests that can identify infected lions. We’ve shown that some of these tests that we’ve developed for lions can be used for cheetahs and across the big cat species. That’s been a real breakthrough because we didn’t have a leopard or cheetah test before.”
SU is taking testing to another level by looking at the epidemiology of the disease. “What’s interesting is that different animals have different levels of resistance to M. bovis,” Miller points out.
She says her team has not been investigating drug resistance in the M. bovis bacteria specifically. “What we are seeing, however, is that over time, the prevalence of animal TB in the KNP and particularly in buffalo is slowly increasing.
“We are looking at the population and systems level, trying to understand what it takes for the immune systems of some animals to clear infection, whether it is minimising animal density or purely genetics.
“This comparative biology enables us to investigate why, sometimes, when an animal gets exposed to TB, it doesn’t develop the disease or, if it does develop the disease, it doesn’t die.
“For instance, we believe that healthy rhinos in the KNP system, not in drought years but in a normal [rainfall] cycle, are fairly resistant to developing the disease. We are now following infected animals and getting samples to see if they can clear infections over time.”
New frontiers
The Animal TB Research Group is using the KNP system as a model to figure out what happens when different infected species interact with each other and with their environment, Miller says. “This can serve as a model for us to go into communities, especially rural communities, where livestock and humans also live in close contact.” The team is also doing work on environmental samples taken from communities living close to protected areas, primarily in KwaZulu-Natal.
“There are literally no statistics about TB in humans caused by M. bovis in South Africa,” Miller points out. “So, we don’t know how big a threat animal TB is to humans in our country.” Recently, SU researchers were involved in a pilot study, done in collaboration with the African Health Research Institute, which suggested that M. bovis DNA could be isolated from people in the area studied.
The researchers are applying surveillance techniques that were developed during the COVID-19 pandemic to track bovine TB in wastewater samples. This work, done in collaboration with the South African Medical Research Council, enables them to monitor a particular community or environment and could help them understand the transmission process and identify the potential downstream risk of TB contamination.
“The problem with TB is that it’s not a one-directional issue,” Miller emphasises. “If we don’t take care of the TB problem in our domestic animals and in our wildlife species, there will be potential spillover into humans, and that will add to the total TB burden.
“We have a unique situation here in the KNP, where [infected] animals are not being treated. What happens in a system when there isn’t intervention? We’re watching as this all unfolds.
“The fact that we have discovered infections in rhinos and elephants in the last seven years or so also suggests that TB is increasing in the environment and in wildlife populations.”
The burden of TB
Tuberculosis, caused by infection with members of the M. tuberculosis complex (MTBC), is considered one of the most significant infectious diseases among animals and humans.
Some MTBC bacteria are specifically adapted to the host they infect, while others, like M. bovis, have a broader range of host species, including humans.
In humans, TB is the leading cause of death from a bacterial infectious disease. About one third of the world’s population is thought to be infected with TB caused by M. tuberculosis. The disease is particularly prevalent in lower-income countries, including large parts of Africa and Asia.
TB is a disease that has been around for millennia among humans. Unfortunately, it is still claiming lives around the world today — in 2021 alone, it was responsible for the deaths of 1,6 million people.
Zoonotic TB is a form of TB that occurs in humans and is mainly caused by M. bovis, which also belongs to the MTBC. M. bovis is the pathogen that causes animal TB, a serious infectious disease affecting both livestock and wildlife populations.
Zoonotic TB accounts for approximately 10% of all human TB cases worldwide. The zoonotic form is primarily transmitted indirectly, through the consumption of contaminated milk, dairy products, or meat.
Sources: World Health Organization and World Organisation of Animal Health
Did you know?
- Wildlife diseases and their implications have become increasingly important in various fields of scientific research, particularly in the aftermath of the COVID-19 pandemic.
- Nearly 60% of all human diseases and 75% of all emerging infectious diseases are zoonotic, meaning they are able to spread from animals to humans.
- Around five new human diseases appear every year. Three of them are of animal origin.
- Animal diseases pose a direct threat to the incomes of rural communities that depend on livestock production.

The research initiatives reported on above are geared towards addressing the United Nations’ Sustainable Development Goals numbers 3 and 15, and goals number 3, 5, and 7 of the African Union’s Agenda 2063.