Studying neurodegenerative diseases on cellular level, and in 3D
Wiida Fourie-Basson
Illustration by Ronel van Heerden | Original image by Dr Sholto de Wet
What if we could tell whether or not a cell is in distress? Or whether the mitochondria in a cell are ‘fit’ or ‘ill’?
In the context of neurodegenerative conditions, wouldn’t knowing that a cell is in distress provide us with an early warning of diseases such as Alzheimer’s and Parkinson’s? After all, in the case of both these diseases, dysfunctional mitochondria are an early hallmark.
Back in 2015, these were the questions that made two specialists — one in the field of cell physiology and the other an electronic engineer — start discussing challenges in the imaging field and how to better work with three dimensional (3D) images of the processes taking place in a cell.
Eight years and two postgraduate students later, Prof Ben Loos from the Department of Physiological Sciences and Prof Thomas Niesler from the Department of Electrical and Electronic Engineering at Stellenbosch University (SU) can say, with confidence, that they have developed a very sensitive method for understanding cellular health.
With this expert pair as study leaders, Dr Rensu Theart developed the mitochondrial event localiser (MEL) as part of his doctoral research on the virtual reality visualisation and analysis of microscopy data. Theart received his PhD in 2021 and is currently a lecturer in the Department of Electrical and Electronic Engineering, where he continues to collaborate with Loos, as well as with the next generation of postgraduate students.

Prof Ben Loos studies the susceptibility of cells to cell death, and the molecular mechanisms that govern this cellular response. He is especially interested in a fundamental cellular stress response termed ‘autophagy’ (from the Greek word for ‘self-eating’). Autophagy is a process by which cells degrade long-lived proteins and damaged organelles. When dysfunctional, this process leads to early cell death (such as that observed in neurodegenerative diseases, including Alzheimer’s and Parkinson’s), but can also assist the cell in staying alive (as in the case of most cancers). | Photo by Stefan Els
Capturing the elusive
According to Loos, there are many tools and techniques to assess the morphological features of mitochondria, such as their number, size, and sphericity. “However, none of these methods adequately addresses the dynamic character of mitochondria. Mitochondria are constantly undergoing movement, with parts of any one mitochondrion joining with other mitochondria, or being pinched off from larger mitochondria. Many of these so-called ‘fusion’ and ‘fission’ events happen in an instant,” he explains.
Not only is it now possible to quantify these two types of events, but one can also do it in the 3D space of the cell.
For Theart, delving into the difficult topic of neurodegenerative diseases means that his work could one day contribute to treatment that would improve the lives of patients in a very direct way: “Some challenges in biology are difficult to resolve from a purely life sciences approach. The small bit that I can contribute in order to accelerate discoveries through cross-disciplinary research fuels me to continue the work, despite challenges.”
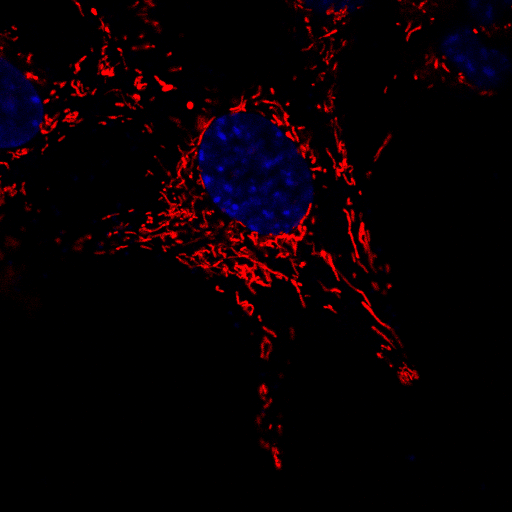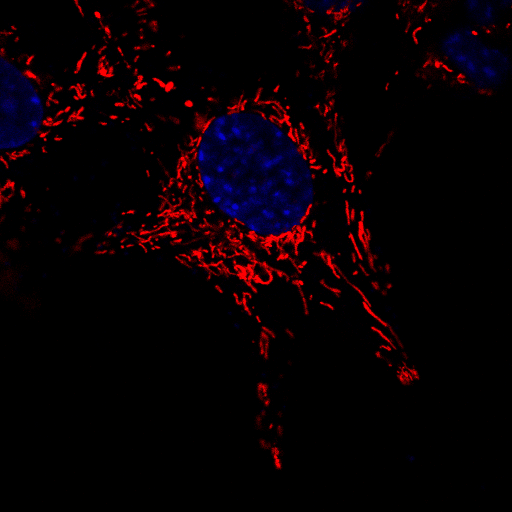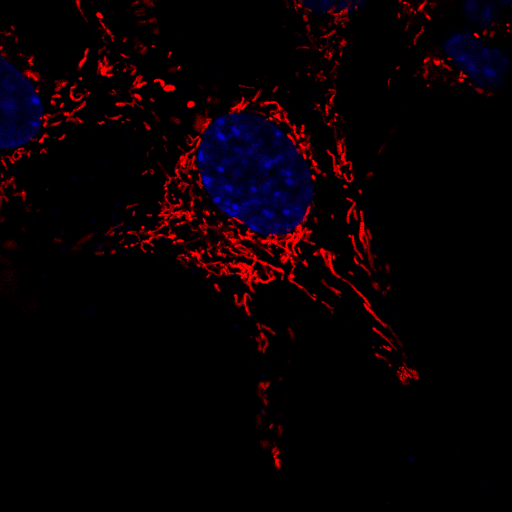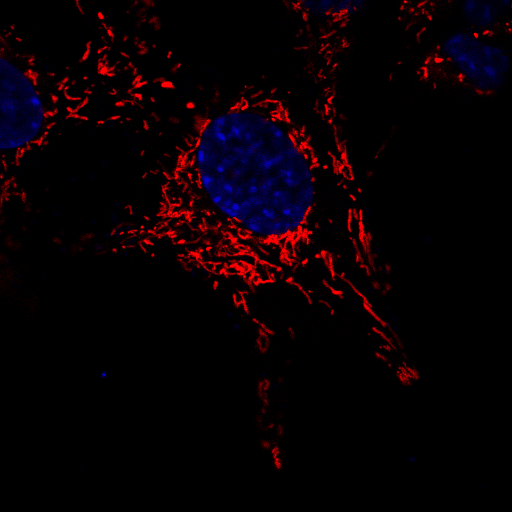
A microscopic image of neuronal cells in which the mitochondria have been stained red in order to observe their movement over a brief period of time. The nucleus of each cell has been stained blue to aid in distinguishing between individual cells. | Image courtesy of Dr Sholto de Wet
What is a neurological condition?
This is any condition that affects the brain, spinal cord, and/or nerves.
What are neurodegenerative disorders?
Such disorders are chronic conditions that damage and/or destroy parts of your nervous system, especially your brain, over time. These conditions are permanent and beyond cure, but many are now treatable thanks to medical advances.
Source: Cleveland Clinic

Dr Sholto de Wet’s work focuses on the manipulation of autophagy in the context of neurodegenerative diseases, particularly Alzheimer’s disease. | Photo by Wiida Fourie-Basson
Unravelling the mechanics of memantine
Theart recently collaborated with one of Loos’ postgraduate students, Dr Sholto de Wet, to better understand, on a cellular level, the effects of the Alzheimer’s drug memantine on autophagy and the mitochondrial network. (Autophagy is likened to a type of recycling system by which cell components, mainly dysfunctional proteins and organelles, are recycled intracellularly.) Memantine is one of the main drugs prescribed for patients with mild to severe Alzheimer’s disease. Yet, despite an 8.5% worldwide increase in the use of the drug between 2008 and 2018, uncertainties remain regarding the most favourable treatment concentrations.
In his doctoral research, De Wet investigated the concentration-dependent effect of memantine in a neuronal cell model. Intriguingly, he found that low (notably, not high) concentrations of memantine led to the induction of mitophagy, a type of autophagy that targets dysfunctional mitochondria through selective degradation — a process that has been shown to enhance cell survival and longevity.
Memantine appears to increase the rate at which such mitochondria are degraded. “This ensures the presence of new and properly functioning mitochondria that contribute towards the cell’s energetic state. When higher concentrations of memantine were used, it was noted that autophagy, a pathway responsible for the degradation of proteins, was increased,” De Wet explains.
Further research is now needed to apply these findings to more complex model systems, including the brain.
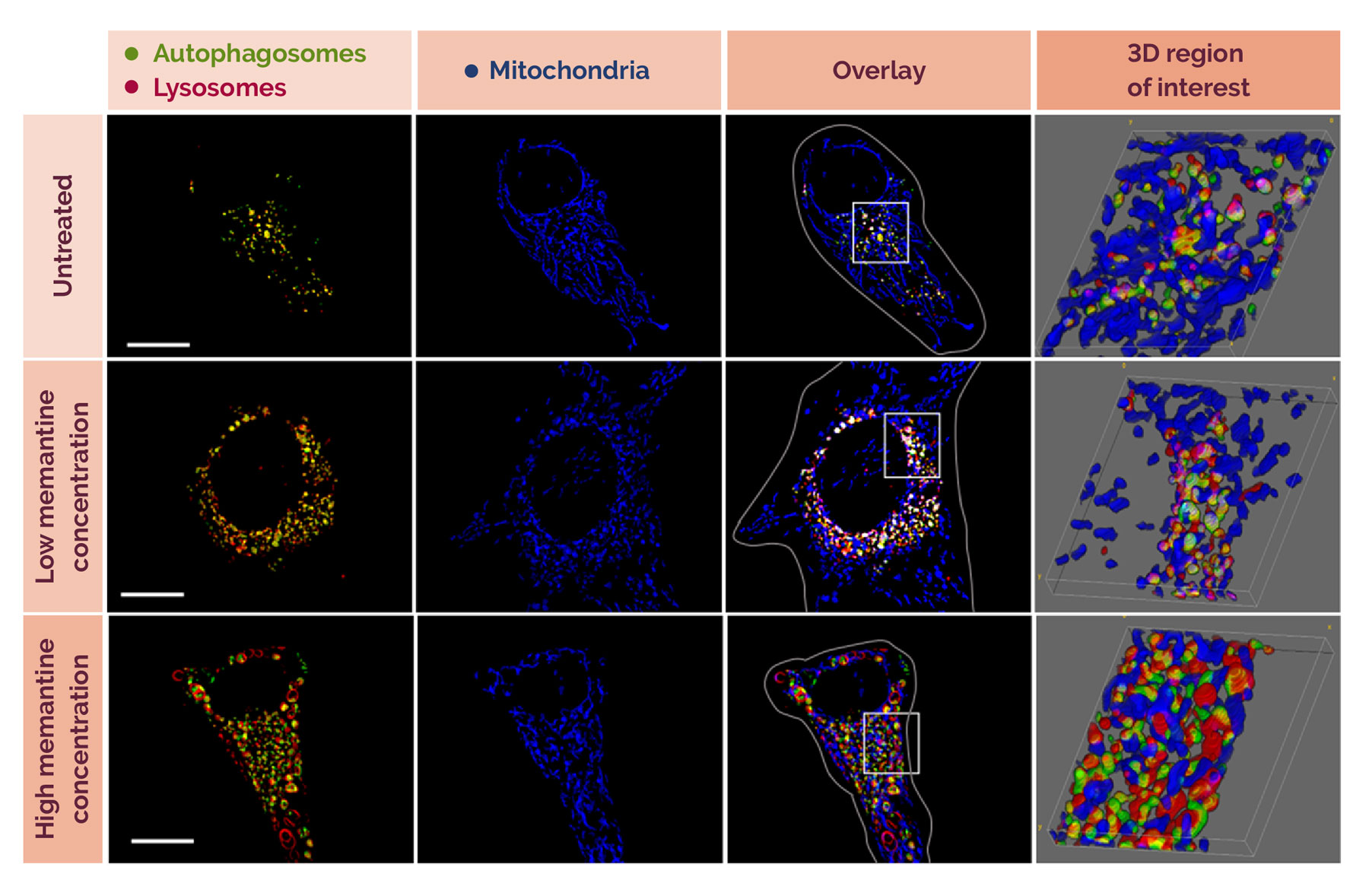
The effect of two different concentrations of memantine on cells. Memantine is one of the main drugs prescribed for patients with mild to severe Alzheimer's disease. Those treated with low concentrations (50 uM) experienced more events (as shown by the presence of more pink and white dots inside the acidic lysosome, indicating that mitochondria are being 'eaten’). At higher concentrations (100 uM), this did not happen to the same extent. The researchers did notice, however, that the ‘mouths’ and ‘stomachs’ of the cells were enlarged. | Image courtesy of Dr Sholto de Wet
Understanding interconnected intracellular processes
Until recently, scientists mostly focused on the insoluble aggregates of misfolded proteins deposited in regions of the brain that are typically affected in the later stages of neurodegenerative diseases. Now, however, the exact process by which these proteins are degraded inside the cell, as well as the role of mitochondria therein, is gaining increasing interest, De Wet says.
Researchers are now starting to understand that there is an interdependent relationship between three important processes (autophagy flux, mitochondrial network function, and lysosomal acidification) in a cell, which De Wet likes to compare to the functioning of a recycling plant: the speed at which a recycling truck is loaded with recyclables and delivered to the recycling plant (the lysosome) is akin to the rate of protein degradation (autophagy flux); the electricity that the plant needs to properly process these recyclables is similar to the energy provided to cells through means of the functioning of the mitochondrial network; and the breakdown of the recyclables is like the acidification of proteins in the lysosome.
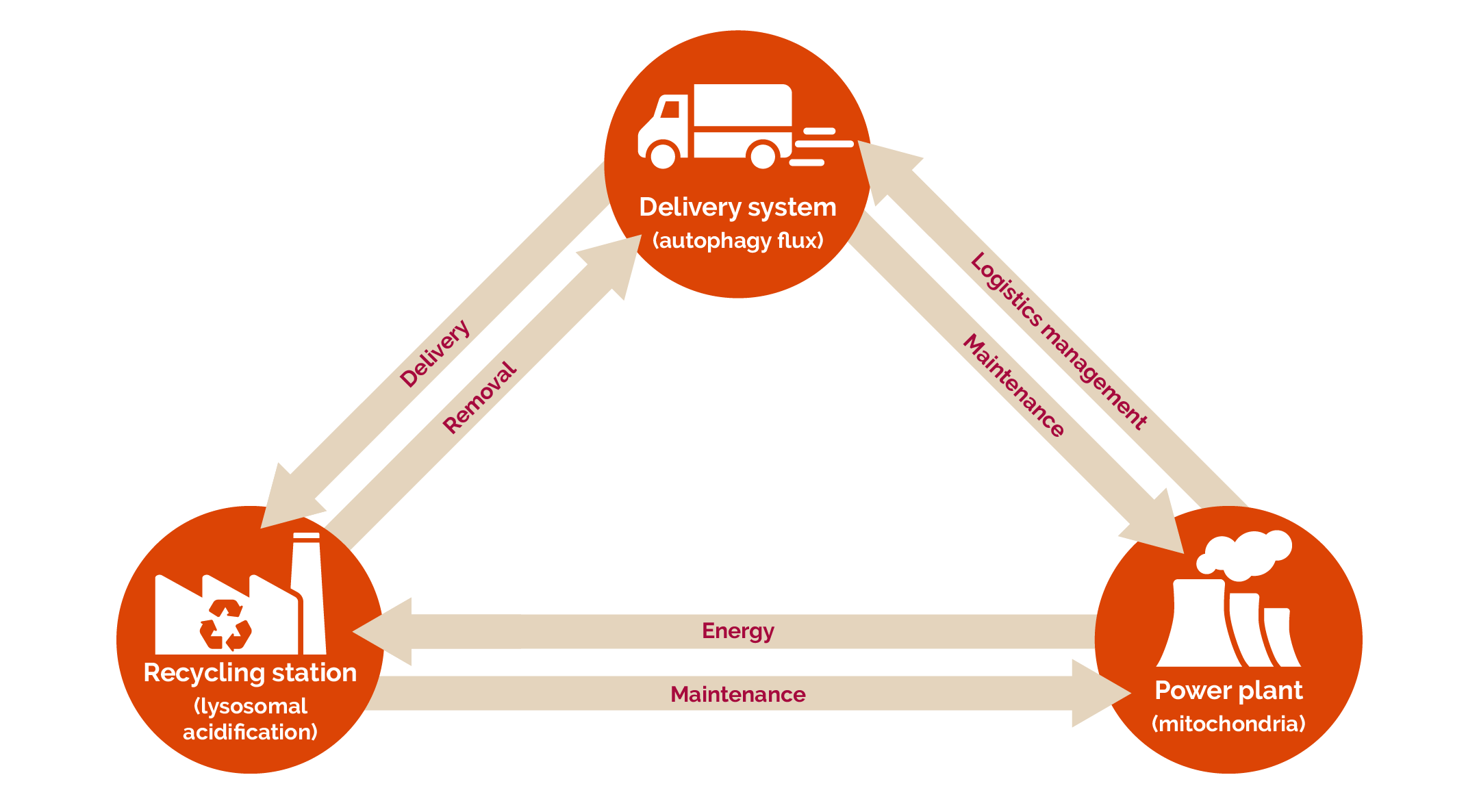
The interdependent relationship between three important processes in a cell — autophagy flux, mitochondrial network function, and lysosomal acidification — can be compared to the functioning of a recycling plant. | Image courtesy of Dr Sholto de Wet
Previously, these processes were studied separately or only in relation to one other, rather than as three interdependent systems. This new way of thinking allows a much better understanding of the molecular defect that drives neurodegenerative diseases.
De Wet and Loos argue that it is not the insoluble protein aggregates that are the root cause of neurotoxicity, but rather the fact that they arise in the first place, due to the dysfunctioning of the protein degradation pathway.
In a co-authored article, De Wet, Theart, and Loos show that the moment the interdependent relationship between the aforementioned three processes is disrupted, the system is thrown out of balance and the problematic proteins aren’t degraded. In the case of neurodegenerative diseases, the proteins will continue to be overproduced and interact with different parts of the cell, including the mitochondria, finally leading to cell death if their levels aren’t suppressed.
This means that targeting any three sides of the triangle, for example by enhancing mitophagy, will protect the cell. “This is important, seeing as many problematic proteins associated with neurodegenerative diseases have been shown to damage the cell by interrupting mitochondrial activity. Memantine may, therefore, offer an avenue by which the mitochondria that are being interfered with can be removed from the system, possibly along with the problematic proteins,” De Wet explains.
“In the context of neurodegenerative diseases, it is known that lysosomes, the recycling stations, don’t work properly. This leads to the building up of material that contributes to the progression of the disease.
“In my thesis, we demonstrated that memantine leads to the reacidification of lysosomes following damage to the mitochondria,” De Wet adds. “This may have been an indirect effect of mitophagy, but to our knowledge this isn’t something that has previously been shown and, as such, deserves attention.”
For Loos, their research points to an important mechanism in the working of memantine that has not been described previously: “If exploited in a well-targeted manner, understanding this mechanism may contribute to a new way of thinking about and approaching the treatment of neurodegenerative diseases.”
Health, disability, and neurological conditions
- According to a recent study, more than 3 billion people worldwide were living with a neurological condition in 2021, with over 80% of neurology-related deaths and health loss occurring in low- and middle-income countries.
- Neurological conditions are the leading cause of ill health and disability worldwide. The overall incidence of disability, illness, and premature death (measured by disability-adjusted life years) caused by neurological conditions has increased by 18% since 1990.
- The number of people living with or dying from disorders of the nervous system has risen dramatically over the past three decades, with 43% of the world’s population — 3.4 billion people — having been affected in 2021.
- Researchers say the rise is due to the global population’s growth, higher life expectancy, and increased exposure to environmental, metabolic, and lifestyle risk factors such as pollution, obesity, and diet.
- The biggest contributors to neurological health loss globally were strokes, neonatal encephalopathy (brain injury), migraines, Alzheimer’s disease and other dementias, and diabetic neuropathy (nerve damage).
- Regions with the highest nervous system burden in 2021 were central and western sub-Saharan Africa. The lowest burden was seen in high-income Asia Pacific and Australasia.
- Access to treatment for neurological conditions varies widely: High-income countries have up to 70 times more neurological professionals per 100 000 people than low- and middle-income countries do.
Sources: The Lancet Neurology, the World Health Organization and Eurekalert.org

The research initiatives reported on above are geared towards addressing the United Nations’ Sustainable Development Goals numbers 3 and 9, and goals number 1, 2, and 3 of the African Union’s Agenda 2063.
Useful links
SU’s Department of Physiological Sciences
SU’s Department of Electrical and Electronic Engineering
A day in the life of a living cell
Research on Alzheimer drug memantine sheds light on novel mechanism in maintaining cell health
Prof Ben Loos’ inaugural lecture (2022)
Prof Ben Loos wants to help improve treatment of Alzheimer’s disease, cancer
Neurological conditions now leading cause of ill-health worldwide, finds study
X (formerly Twitter): @MatiesResearch and @StellenboschUni