How to manage your orthopaedic patient who has chronic renal failure
Chronic renal failure
Definition CRF:
Irreversible and progressive reduction in the glomerular filtration rate (GFR) to below 25% of normal for at least three months.
Problems in CRF:
20 % of dialysed patients have bone pain.
Causes CRF in Children:
< 5 yr.:
In children the causes of chronic renal failure are mainly congenital.
Causes in adults
Pre Renal
Renal
Post Renal
Pathophysiology
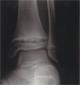
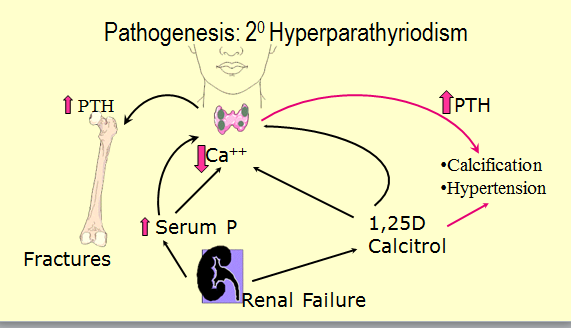
Renal Osteodystrophy develops because in CRF both calcium and phosphate metabolism are abnormal. This results in elevated parathyroid hormone levels. The bone turnover is further interfered with by high aluminium levels from the dialysis water and from oral phosphate binders, given to treat the elevated phosphate.
Dialysis only filters out the electrolytes – the plasma proteins remain abnormal. Haemoglobin breakdown increases levels of Iron beta 2 microglobulin. This ultimately forms amyloid in the tissues. In other words, despite good dialysis, amyloidosis will eventually develop from blood breakdown proteins, amongst others.
Metabolic and other changes
Metastatic carcinoma is common due to the heavy immunosuppression needed to counteract rejection after a renal transplant.
Vitamin D Metabolism. Sunlight changes 7 dehydrocholesterol to cholecalciferol in the skin (occurs in hair or feathers in other animals, hence licking or preening).
The liver converts cholecalciferol to 25-hydroxycholecalciferol. In the normal kidney this is converted into the active form 1,25 dihydroxycalciferol.
This acts to regulate blood calcium, promoting the healthy mineralization, growth and remodelling of bone.
In kidney disease this last step in the final pathway (of Vit. D metabolism) is deficient!
Bone histology in CRF
Seen in 5 to 30% dialysed patients. Irregular trabeculae, irregular remodelling sites. Numerous osteocytes. Thickened osteoid seam.
Profound decrease in the number of active remodelling sites. ":Adynamic bone disease" i.e. few osteoid seams.
Caused by hyper parathyroid and defective mineralisation without decreased bone formation.
Not always adynamic - it can superimpose any of the above described groups. PTH seems to increase uptake of Al into bone. Aluminium interferes with the mineralisation process.
Prevention of Hyperparathyroidism
The biggest challenge it to get the patient to stick to a low protein (no meat al all) diet. Dairy products, a possible source of Vitamin D are also banned!
Causes of Joint Pain in CRF
CRF patients are immunologically compromised and susceptible to septic arthritis. Poor renal function results in uric acid buildup and gout. Avascular necrosis is due to steroids either used to treat immunological CRF or to suppress rejection in the patient already having had a transplant.
Dialysis Arthropathy. Poly arthritis is common. CRP raised, weakly positive Rheumatoid factor. Polyarthritis. Common (36 of 97)
Causes joint pain including morning stiffness.
Have high C-reactive protein, weakly positive Rheumatoid factor.
X-ray changes: Periarticular erosions. Intra articular calcification or an erosive arthritis.
Joints: Shoulder, hips hands , knees, wrists.
Pathology.: Amyloid and hemosiderin deposits.
Is a common cause of entrapment neuropathies. Carpal tunnel syndrome is commonly associated with amyloid deposits.
Avascular necrosis in CRF
Common in CRF because:
Steroids are the main cause of the high incidence of AVN in patients
with chronic renal failure. They are often prescribed for the underlying
cause of CRF which is commonly autoimmune. Once the patient has had a
transplant he is put on steroids to suppress rejection. Although his renal
function may improve he has a high risk of avascular necrosis. 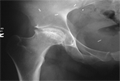
AVN is common after renal transplants. Jagose states the incidence to be between 3 and 41%
Steroids are the main cause of the high incidence of AVN in patients with chronic renal failure. They are often prescribed for the underlying cause of CRF which is commonly autoimmune. Once the patient has had a transplant he is put on steroids to suppress rejection. Although his renal function may improve he has a high risk of avascular necrosis.
AVN is common after renal transplants. Jagose states the incidence to be between 3 and 41%
Common Presentations of Chronic Renal Failure
Common clinical presentations of chronic renal failure include anaemia, growth retardation, urinary complaints, hypertension and mental irritability. Renal osteodystrophy is a common long-term complication of end stage renal disease.
Insomnia is a common complaint.
