Intermolecular interactions
Intermolecular interactions in solid state properties and phenomena
Research Fields
Structural chemistry, computational chemistry, crystallography, physical chemistry
Research Highlights
- Gold complexes as hydrogen- and halogen-bond acceptors
- Interactions between gas molecules and porous materials
- Solid state properties of pharmaceuticals
Research Summary
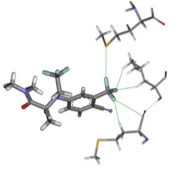
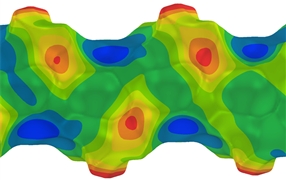
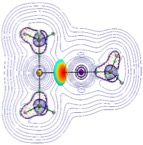
The focus of the Esterhuysen group is the analysis of non-covalent interactions between molecules and ions in the crystalline solid state, to understand their origin and eventually harness them in the design of novel materials with interesting properties, especially porosity. In particular, we concentrate on unusual interactions, such as aurophilicity (Au···Au), as well as halogen bonds. The ubiquitous hydrogen bond is, nevertheless, not ignored, although we generally focus on unusual aspects of hydrogen bonding, such as gold as a hydrogen-bond acceptor. We utilise a combination of experimental crystal structures, through “data-mining” of the Cambridge Structural Database, and computational methods.
Applications of Research
- Catalysis
- Gas and vapour separation
- Drug formulation

Catharine Esterhuysen
Professor of Physical Chemistry
ce[at]sun.ac.za
+27 21 808 3345